Career Advice
Information about how to get involved in research as a trainee
Good Clinical Practice
At a trainee level, the NIHR Good Clinical Practice module can help with understanding of sound, basic research principles - it is free to complete & a certificate of completion is provided. Holding an up-to-date GCP certificate is a CCT requirement for all general surgery trainees.
NIHR API Scheme
The NIHR Associate Principal Investigator (API) scheme offers trainees the opportunity to be involved in a nationally recruiting study or Randomised Controlled Trial under the guidance and support of a local Principal Investigator. The aim of the scheme is to teach trainees about study recruitment and help develop the Principal Investigators of the future. Click here to find out more.
For a list of registered studies open to applications for the API scheme with relevance to breast disease, please click here and search for “breast” in the study title column
ABS Badged Studies
The following ABS badged studies are signed up to the API Scheme. Find out more below.
Out of Programme Trainee Experience
Watch the video below to find out about out of programme research experience, from a trainee perspective and important things to consider when exploring this option. Fast forward to 4:02:58 to hear Mitali’s talk.
Academic Careers Advice
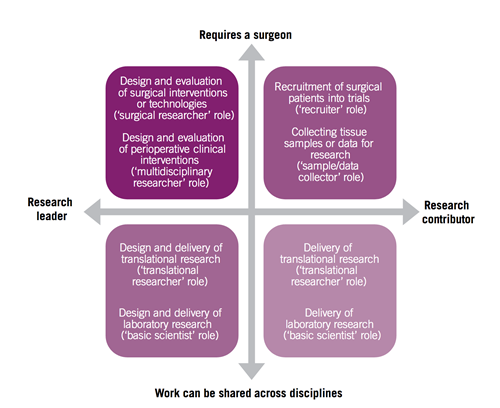
Increasingly there is an expectation – quite rightly – that participation in clinical research should be an integral part of our practice as consultant breast surgeons. Clinical research allows us to create the evidence base we need to inform our clinical practice, while giving our patients the opportunity to participate in clinical trials of new treatments or technologies. Given that surgeons often act as leaders within their clinical teams, there are many opportunities for surgeons to contribute to research, and these are perhaps best summarised in Figure 1.
Figure 1: From NCRI, Challenges and opportunities in surgical cancer research in the UK. October 2012
Getting involved in clinical research may be a daunting prospect, particularly for those with little or no previous experience in this area. For many, particularly newly appointed consultants, a first step may be to open or develop a portfolio of clinical trials within their unit (the ‘recruiter’ role in Figure 1). In this context, it’s important to recognise that clinical research is a team game, and it’s crucial to make sure that you have the support of your colleagues within the MDT. Many trials are multidisciplinary, and rely on the expertise of a variety of team members for successful delivery – the radiologists for collection of biological samples (such as tumour core biopsies) or the oncologists, for delivery of some systemic therapies in neoadjuvant or peri-operative studies. Experience suggests that it’s always better to get everyone on board before you start. It may also be more productive to try and open one or two trials in areas of real interest locally, where you feel that you will be able to recruit productively, than to try and open multiple trials and have limited recruitment, which can be frustrating and demoralising for everyone.
When it comes to negotiating your local hospital’s research governance procedures, or finding clinical research nurse (CRN) support “on the ground”, support is available through the National Institute of Health Research (NIHR) Clinical Research Network. The NIHR supports the setup and delivery of clinical research within the NHS through a network of Local Clinical Research Networks. Making contact with your LCRN will allow you to access the expertise and support necessary to deliver a trial within your unit. Most of the multicentre trials in breast surgery, such as POSNOC and LORIS, have been adopted onto the National Institute of Health Research (NIHR) Trials Portfolio and thus are eligible for support through the LRCNs. In my experience, a good working relationship and clear communication with your CRNs is essential to successful trial recruitment!
Some individuals may wish to extend their level of academic or research activity into some of the other roles outlined above, or indeed may be newly appointed academic consultants facing the challenges of reconciling the demands of a consultant post and a university appointment. Although tricky, there are a few things that I have found helpful over the last couple of years since taking up an academic post.
Having a job plan which allows the integration of these two aspects of an academic post is key to this. Having sufficient “chunks” of time to allow uninterrupted writing or data analysis is essential – and it’s important where possible to arrange a job plan that will accommodate this. However, I have found that maintaining some degree of separation of clinical and academic activity is necessary for academic productivity, while allowing me to concentrate on clinical issues on the days that I’m in the hospital. Having said that, a level of flexibility is sometimes necessary to facilitate the travel that’s often required in an academic post – whether to conferences, investigator meetings or other commitments away from home. This in turn means that it’s necessary to pay fairly close attention to both the diary of clinical commitments such as out-patient clinics and operating lists – as well as the on-call rota – to make sure that everything is covered (it sounds obvious but can rapidly become complex and challenging!).
Being able to manage deadlines (whether they are grant application deadlines, conference submission deadlines, or 31 day targets) can also prove daunting – I have found that keeping a well-ordered “to-do” list with deadline dates has been very helpful for my sanity. Linked to those deadlines are what seem like the interminable waits for feedback – so patience is vital; the research world proceeds at a different pace to the operating theatre. A thick skin is also a necessary attribute, as inevitably there are rejections and disappointments along the way. Having an experienced, senior mentor whose advice and input you trust can be invaluable, both in these situations, and in providing more general guidance as you develop your academic career. They may also be able to help you retain a focus on your key areas of interest and help you to recognise things that may detract from your key targets – learning to say “no” is never easy but it’s an essential skill, especially when you’re starting out. Maintaining a focus on “your” area of research can be difficult in view of all the competing and conflicting demands on your time, but it is vital.
With all this said, there is no doubt that incorporating academic and research activity in a consultant career – although challenging – can prove extremely satisfying. For me, some of the rewards include the continued intellectual stimulation of gaining new knowledge and skills, as well as the opportunities for multidisciplinary interaction that arise from research activity. Also, the blend of clinical activity, research (both clinical and translational) and educational activity that comes from an academic role allow a greater variety to the job than might be the case with a full-time NHS clinical consultant post.
Finally, although there may be challenges and obstacles, the most important qualities for success in integrating academic and research activity into a consultant career are probably enthusiasm and determination, with which almost anything should be possible!
Written by Stuart McIntosh
Consultant Breast Surgeon, Queen's, University Belfast
This pathway is for trainees who aspire to follow a clinical academic career path. It is delivered as a partnership between Universities, Deaneries and the NIHR Trainees Coordinating Centre. NIHR Academic Clinical Fellowships (ACFs) allow medical and dental trainees to undertake 25% research and 75% clinical training over 3 years. This period of research is classically used to develop a research portfolio that would allow competitive application for a PhD fellowship from one of the academic funding bodies such as NIHR, Medical Research Council or Cancer Research UK. Clinical Lectureships (CLs) allow trainees to undertake 50% research and 50% clinical training over 4 years, with the aim of developing post-doctoral research, before the award of a Clinician Scientist Fellowship or Senior Lectureship.
A Clinical Academic is a clinician who also wants to include an element of research, teaching or management in their role with the aim of improving clinical practice within their speciality. Medical clinical academic training pathways have become more structured. The academic career path can begin at medical school with an intercalated BSc or MSc degree or MB PhD programme, alongside other opportunities to be involved in research. Nevertheless, the move into an academic programme is possible at various stages in postgraduate medical training.
Recognising the value of supporting clinicians to do research in their area of expertise, the National Institute for Health Research (NIHR) provides a range of schemes to encourage aspiring Clinical Academics. Academic foundation programme posts funded by Health Education England (HEE), offer protected academic time during Foundation Year (FY2) training, usually in the form of a four-month block spent away from clinical practice. The NIHR Integrated Academic Training (IAT) Pathway supports Academic Clinical Fellowships (ACFs) for up to three years enabling academic training alongside specialty training with the aim of supporting entry into a PhD programme. The NIHR also provides Doctoral Research Fellowships (DRFs) supporting a period of out-of-programme leading to a doctoral degree (PhD), and finally, Clinical Lectureships (CLs) of up to four years’ duration enabling post-doctoral clinicians to split their time equally 50:50 between clinical and academic work (Figure 1a). Universities are required to provide one matched CL post per NIHR-funded CL post through local funding. DRFs are personal awards following a competitive application process. Equivalent personal funding to pursue a substantive period of research leading to a higher degree may be obtained from the Medical Research Council (MRC), Wellcome Trust, British Heart Foundation and other charities.
At Consultant level, the NIHR offers Senior Clinical Lectureships or Advanced Fellowships as well as prestigious senior Research Professorship awards. Equally, there is the opportunity to use NIHR or equivalent funding to pay for research sessions within a Consultant job plan.
NIHR Integrated Academic Training Programme in England
For overviews of the IAT pathways for doctors, see Integrated Academic Training Pathway (Medicine).
In Scotland, the Academic Foundation Programme (AFP) supports a dedicated four-month academic block for research or medical education activities. As well as acquiring clinical competencies expected of all trainees, each academic foundation trainee is allocated a personal academic mentor who is actively engaged in promoting and undertaking research at University and/or NHS level. The AFP provides a great opportunity to become more involved with medical research, education and/ or management.
NHS Scotland | Scottish Medical Training Foundation Programmes
All four Scottish Medical Schools in conjunction with NHS Education for Scotland (NES) are offering additional Clinical Academic Training opportunities (ACAT/DCAT/ECAT/GCAT Programmes). These early academic career programs are focusing on core trainees (CT1/2) or early years of run-through specialty trainees (ST1/2). Over a two-year programme, the appointed academic trainees will be given an academic mentor, access to the full support of the academic infrastructure, a small budget for research/publications and travel to conferences, help with applying for fellowships and identification of research opportunities. These trainees will also be encouraged to formulate plans for medium to long-term research opportunities which may include personal fellowship applications, MD/PhD studentships, or entering into training programmes with the view of progressing towards competing for SCREDS Clinical Lecturer positions.
- ACAT Aberdeen Clinical Academic Training
- DCAT Dundee Clinical Academic Training
- ECAT Edinburgh Clinical Academic Training
- GCAT Glasgow Clinical Academic Training
The Scottish Clinical Research Excellence Development Scheme (SCREDS) provides an integrated training and career development pathway enabling clinicians to pursue concurrently or sequentially academic and clinical training within the NHS. It facilitates both the attainment of a senior clinical academic appointment and the award of a Certificate of Completion of Training (CCT). Normally time within the SCREDS appointment is split into 20% for research training and 80% for clinical training. These posts are funded either directly by universities or by NES.
NHS Scotland | Scottish Academic Training (SCREDS)
At Consultant level, NHS Research Scotland (NRS) supports NHS clinical staff in developing a research career by funding protected time to contribute to, conduct and lead clinical research, in order to increase capacity in areas that are either aligned to research excellence locally or nationally or areas where the potential exists to develop research excellence. The Scottish Senior Clinical Fellowship scheme supports clinical scientists in their transition to leadership and provides a research-focussed entry point to a permanent clinical academic career for a period of five years.
NHS Scotland | NHS Research Scotland Fellowships
The other devolved nations also support integrated academic training programmes and details may be found on the following websites:














